Atomic Physics employing Laser Spectroscopy:
Introduction
The
main focus of the theoretical program of Dr. Drake and coworkers at the
For example, isotope shifts from laser resonance experiments can be used to measure nuclear radii to an accuracy exceeding that of traditional high-energy nuclear scattering methods.\A0 The principle of the method is to calculate all contributions to the isotope shift with sufficient accuracy and reliability that the residual energy shift due to finite nuclear size can be determined from the comparison between theory and experiment.\A0
The first successful demonstration for the radius of 3He relative to 4He [35] has motivated to extend this sort of measurements to probe the nuclear charge radius in 8Li, 9Li, 11Li.\A0 The 11Li case is particularly interesting because it has a halo-nucleus with a 9Li core and a neutron pair circulating around it. A comparison of the charge and mass radii would provide important information on this unusual structure.\A0 Its half life of only 8.7 ms and low abundance make conventional scattering measurements difficult.\A0
The proposal
is to measure the isotope shift in the 2S - 3S two-photon transition by laser
resonance and compare with our theoretical value, including relativistic and
QED contributions.\A0 All the tools are now in place to perform these
calculations to the necessary accuracy [36].\A0 A recent measurement of the 6Li - 7Li isotope shift
[37] confirms the correctness of our results.\A0 There are many other transitions involving the
heavier He-like and Li-like ions that could be studied with the unique opportunities
given by the TITAN facility at TRIUMF. In the following the experimental approaches
will be described in more detail.
Laser-spectroscopy on singly charged Li ions in a trap
Comparison of experimental with theoretical Li+ fine and hyperfine energy intervals provides a stringent test of our understanding of two electron systems. In particular, information is obtained about quantum electrodynamic (QED) contributions that scale to lowest order as Z4 a3 times the Rydberg energy, where Z is the nuclear charge and a is the fine structure constant.\A0
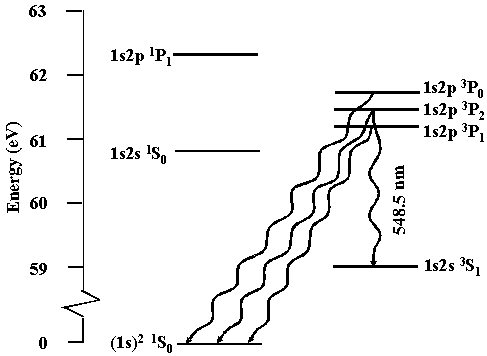
Precision spectroscopic measurements of isotope shifts also determine nuclear radii more accurately than is possible using nuclear scattering.\A0 Lithium has two stable isotopes 6,7Li and three unstable isotopes 8,9,11Li. The low lying Li+ energy states are shown below.\A0 In particular, Li+ has a 1s2s 3S1 state having a lifetime of 59 \B1 13 sec.\A0 Precision laser spectroscopic measurements have been done using the 1s2s 3S1 \AE 1s2p 3P0,1,2 transition that occurs at a wavelength of 548.5 nm where narrow linewidth ring dye lasers readily operate. Recent measurements have been limited to an uncertainty of about 0.5 MHz by the velocity spread of a Li+ beam.
We
therefore propose inserting Li+ in an ion trap and applying laser
cooling techniques to reduce the ion velocity [39].\A0
The Li+ 1s2s 3S1 metastable
state has been successfully loaded into an ion trap [40].
Laser cooling should be possible as the 1s2p
3P state has a lifetime of 44 ns and decays primarily to the 1s2s
3S1 state.\A0 The
largest branching rate to the ground state (1s2p 3P1 \AE
(1s)2 1S0) has been
 estimated
to be 1.787 x 104 sec-1 [41].\A0 The remaining branching rates (1s2p 3P0,2 \AE (1s)2
1S0) are suppressed by several orders of magnitude due
to violation of additional selection rules. \A0Hence, laser
cooling should be possible by detuning the laser frequency slightly below either
of the 1s2s 3S1 \AE 1s2p 3P0,2 transitions. The proposed ion trap will permit the
study of the stable and unstable Li isotopes.
estimated
to be 1.787 x 104 sec-1 [41].\A0 The remaining branching rates (1s2p 3P0,2 \AE (1s)2
1S0) are suppressed by several orders of magnitude due
to violation of additional selection rules. \A0Hence, laser
cooling should be possible by detuning the laser frequency slightly below either
of the 1s2s 3S1 \AE 1s2p 3P0,2 transitions. The proposed ion trap will permit the
study of the stable and unstable Li isotopes.
The work on 6,7Li+ will not only yield results of inherent
interest but will also test/prepare the ion trap for studies of radioactive
ions produced at TRIUMF as well as train students.\A0 Lithium ions can be generated by collision of
an atomic beam with an electron beam. The electron excitation results in about
0.1% of the ions occupying the 1s2s 3S1 metastable state.
If the on-line sources of ISAC are employed, higher temperatures can be used, resulting in a significant increase of the fraction in the metastable state. Light produced by a commercial ring dye laser would then be used to laser cool and probe the ions.\A0 The ultimate resolution of the measurements will be limited by the natural linewidth of the 1s2p 3P state which is only 3.6 MHz.\A0 Hence, even a 1% determination of the line center would improve the 1s2p 3P fine and hyperfine structure splittings by more than an order of magnitude.\A0
Isotope shifts would be found by measuring absolute transition frequencies using the so called femtosecond frequency comb technology. The measurements program would clearly start with off-line sources and stable isotopes, and in a later stage, when all necessary tools are developed, could be extended to the short-lived species where the production yield, for example for 11Li, is more than an order of magnitude higher than at ISOLDE.
